To understand biology, and what causes disease, we seek to understand life at every scale — from the minutiae of protein interactions to the ways disease affects entire populations. Imaging is at the heart of all of this research; new discoveries are limited by what we’re able to see within biological samples. Pinpointing one protein in a swarm of thousands, or watching how cells migrate over time as an organism develops, however, can come with a variety of challenges.
In April, we announced our four scientific grand challenges in service of our goals at CZI, one of which is to “develop novel imaging technologies to map, measure and model complex biological systems.”
Two powerhouses in their fields, the Imaging Institute and San Francisco Biohub, are coming together in service of this new grand challenge. With some of the brightest minds in biology, imaging technology and software development, we can continue to fill technological gaps and enable world-changing research.
Our co-founder and co-CEO Dr. Priscilla Chan recently met with leaders guiding the formation of the new Biohub, Drs. Scott Fraser, Sandy Schmid, Stephani Otte and Matthias Haury, to talk about progress on building the new institute, and about its mission.
“We have a real opportunity to bring together the best of both worlds,” said Fraser, president of the Imaging Institute, during the talk. “Imaging is one of the few things that can link across all the time and space dimensions that we care about.”
Science across scales and disciplines
Pushing the bounds of imaging isn’t new for the San Francisco Biohub and Imaging Institute. Both organizations have already taken down barriers to research by building imaging tools that don’t exist anywhere else, as well as creating pioneering cell atlases that have redefined how we understand health and disease.
“We have a real opportunity to bring together the best of both worlds.
Scott Fraser, president of the Imaging Institute
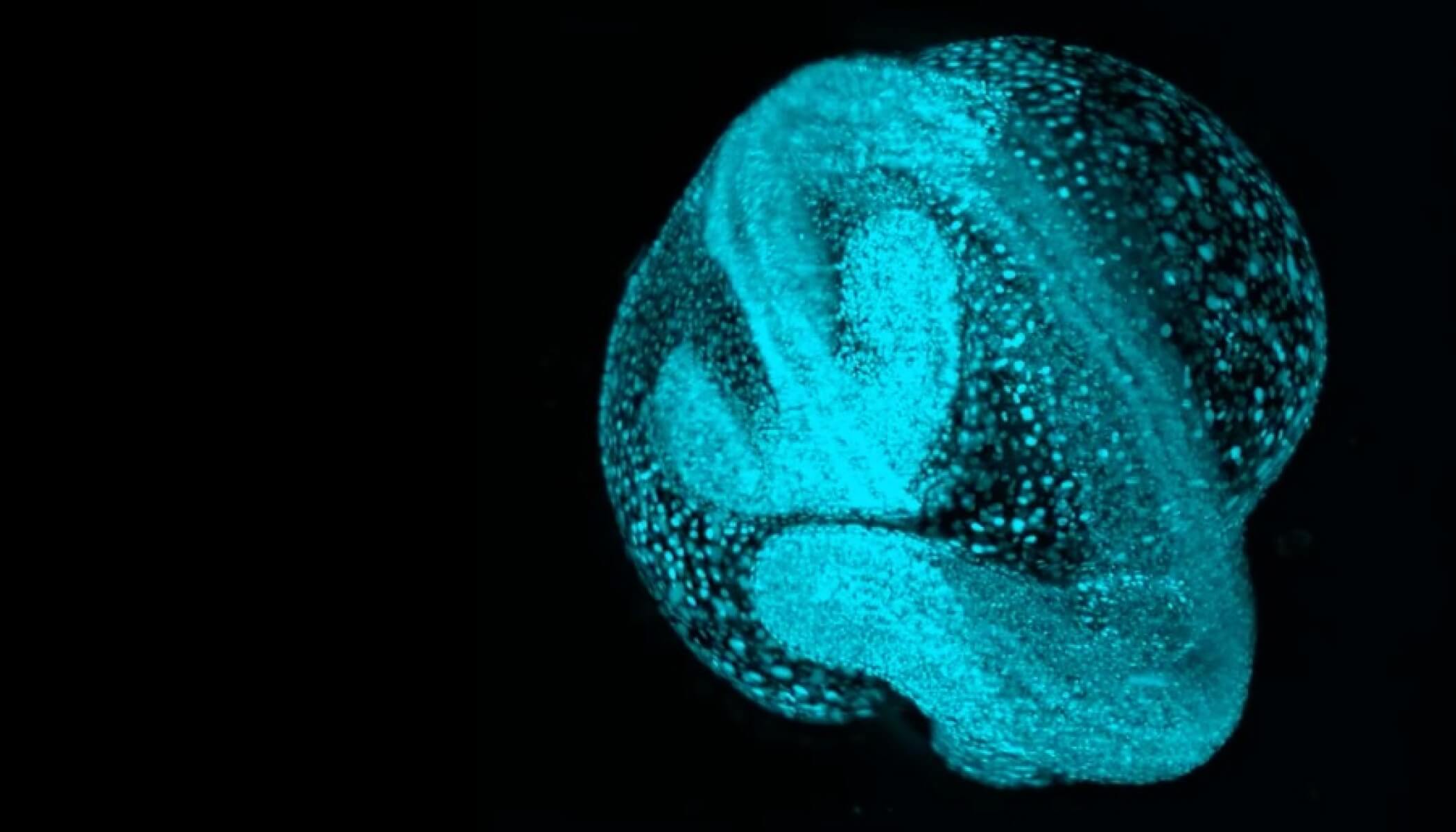
One example is the San Francisco Biohub’s research on how zebrafish embryos develop over time. In order to capture video images of whole zebrafish embryos through various developmental stages, Biohub scientists built a custom microscope, along with novel software that can find and track the movement of each cell in the videos. The “Google Earth” of embryology resulting from this research is Zebrahub, which brings a new vision to developmental biology and helps us understand our own cellular origins.
Projects like Zebrahub require scientists from a host of different disciplines. Teams across the Biohub, along with interdisciplinary partners, worked to build the microscope, develop the cell tracking software and interpret the resulting images. The collaborative nature of this project isn’t unique to our research on zebrafish — it’s part of our philosophy, and we believe collaboration is critical to drive scientific advancement in all of our work.
Affecting science worldwide
Because understanding the mechanisms of disease is a complex problem, it requires a multidisciplinary solution. One example of this at work is the laser phase plate, a collaboration between lead researcher Dr. Holger Müller of University of California, Berkeley; Thermo Fisher Scientific, and the Imaging Institute. The laser phase plate is a piece of hardware that can be inserted into an electron microscope, and enables scientists to see the detailed inner workings of cells with more clarity and contrast than ever before. By partnering with the Imaging Institute and Thermo Fisher, Holger’s work can be refined and eventually shared out with the global community, expanding the reach of the tool significantly.
“This is really going to start to open up the whole cellular environment,” says Otte, vice president of imaging science. With such a detailed look at cells, we’ll be able to understand how their functions might change during disease, helping us find opportunities to develop new treatments.
Other examples of global, interdisciplinary collaboration are the Organelle Profiling project and the Organelle Box, both from the San Francisco Biohub. This organelle work has shed light on the proteins that make up the cellular compartments that carry out cellular functions necessary for life, and how those functions change in response to infection or disease.
“We’re doing things that nobody else has tackled.
Matthias Haury, COO of the Imaging Institute
The Organelle Box contains cell lines with some 30 labeled organelles, which can be shipped to researchers upon request. The data and methods underlying both projects are available to scientists around the world, who can use these tools to explore the questions of most interest to them. And as the researchers who use these tools share their results with one another and with CZI, we’ll pave the way for open discussion among groups and institutions.
“This opportunity for collaboration really broadens the impact of our work,” Schmid, vice president of integrated biology, says.
Solving CZI-shaped problems
As the teams join together for this new grand challenge, they will continue fostering a culture of collaboration and learning by taking on new projects to push the bounds of imaging, as well as supporting ongoing projects. These big-bet, multidisciplinary projects are what we call “CZI-shaped problems” — niches that we believe we can drive significant impact in.
“We want to do science that passes the deletion test,” Fraser says, “science that wouldn’t get done if we didn’t do it.”
Finding and taking on these problems is how we’ll make strides toward our imaging grand challenge, and our goal of curing all disease.
“We’re doing things that nobody else has tackled,” adds Haury, COO of the Imaging Institute. “We have a unique role in the world of science to take on.”